On March 21, Nature News’ homepage featured a report on the groundbreaking occurrence of the world’s first pig liver transplantation into a human body, sourced from Xijing Hospital of the Air Force Medical University of PLA.
David Cooper, a xenotransplant immunologist at Massachusetts General Hospital in Boston, congratulated Dou’s team on the major achievement. The research conducted by the Chinese team lays the groundwork for gene-edited pig liver transplantation, offering crucial insights into its potential to sustain human life, said Cooper.
The transplanted organ exhibited normal function for a period of 10 days, showing no signs of hyperacute rejection.
On March 10, under the leadership of Dou Kefeng, an academician of the Chinese Academy of Sciences, and Tao Kaishan, director of the Department of Hepatobiliary Surgery, Xijing Hospital of the Air Force Medical University of PLZ achieved a successful auxiliary whole liver transplantation from a polygenic-edited pig to a brain-dead patient.
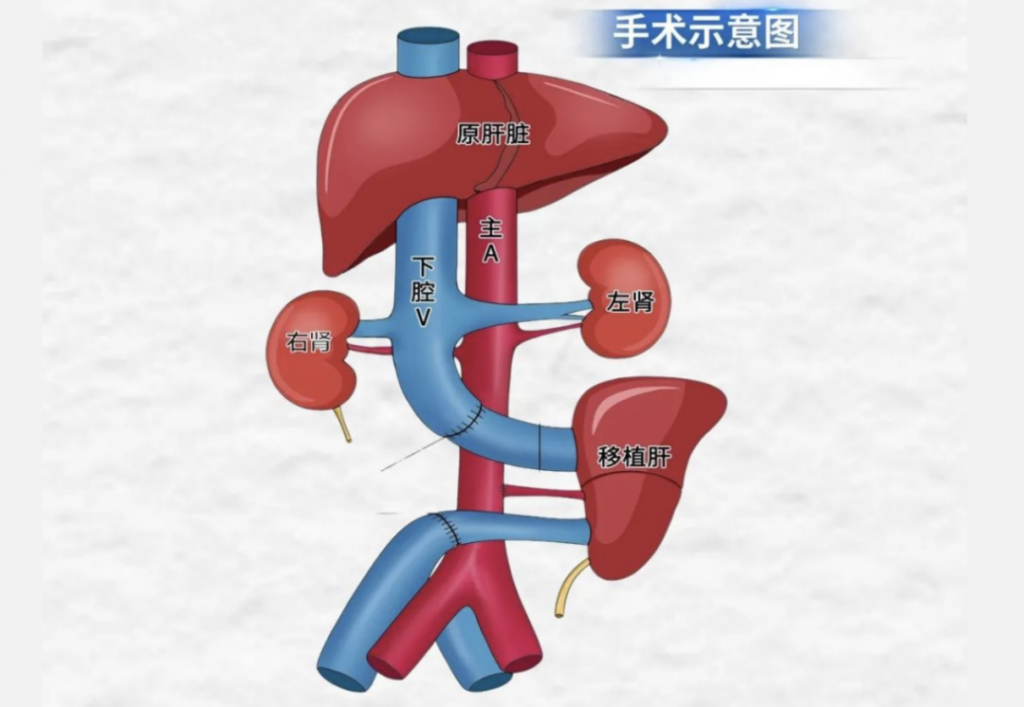
The recipient was a brain-dead patient, specifically one with severe closed craniocerebral injury, confirmed as brain-dead through three evaluations. Their family consented to participate in xenogeneic liver transplantation research without charge. The procedure involved auxiliary liver transplantation, preserving the recipient’s own liver, mimicking supportive care for clinical liver failure patients.
The donor pig had three porcine antigens associated with hyperacute rejection and acute humoral rejection deleted, while two human complement regulatory proteins and one human thrombomodulin were introduced.
During the operation, the transplanted liver began secreting bile immediately upon restoration of blood flow, with no observed hyperacute rejection. Twenty-four hours post-operation, the recipient exhibited stable hemodynamics, the transplanted liver showed robust bile secretion, and B-ultrasound confirmed adequate blood supply to the transplanted liver without any signs of rejection in puncture pathology.
The study concluded on March 20 at the family’s request, after the transplanted liver had functioned for 10 days. Post-termination, the transplanted pig liver exhibited healthy texture and normal coloration.
Dou Kefeng, an academician of the Chinese Academy of Sciences, hailed the study as a major breakthrough in xenogeneic organ transplantation, marking a significant stride toward clinical application. He emphasized its role in providing both theoretical framework and empirical data for future clinical endeavors.

Professor Tao Kaishan of Xijing Hospital of Air Force Medical University of PLA highlights three pivotal questions addressed by this research breakthrough:
The use of brain-dead recipients in clinical trials of xenogeneic organ transplantation provides a more relevant model for assessing transplanted organ function and evaluating the efficacy of pig gene editing strategies. This approach also facilitates the accumulation of valuable clinical experience compared to using animal recipients such as baboons and macaques in preclinical research.
In cases of acute fulminant liver failure, where liver transplantation stands as the sole life-saving option, xenogeneic liver transplantation serves as a temporary measure until a suitable homologous liver becomes available. Alternatively, it aids in restoring the function of the patient’s own liver. This underscores the significant clinical utility of xenogeneic liver transplantation, prompting the adoption of auxiliary liver transplantation in this study.
The immunosuppressive regimens optimized in animal studies may not directly translate to human recipients. By employing brain-dead patients as transplant recipients, clinical immunosuppressive drugs can be utilized, thereby enabling the derivation of an optimal immunosuppressive strategy tailored specifically for clinical xenogeneic organ transplantation.
Earlier in January, a team from the University of Pennsylvania achieved the first successful in vitro liver perfusion using a gene-edited pig liver, sustaining function for three days. This latest achievement, the first-ever transplantation of a gene-edited pig liver into a human body, was lauded by Nature News as a milestone in the transplantation of animal organs into human recipients.
(Source: Nature, VCG)



