On 15 September, the international medical academic journal, the Lancet infectious diseases, officially published the research results of the Beijing Institute of Biological Products of China National Biotec Group (CNBG), showing that the Chinese COVID-19 inactivated vaccine is safe with high tolerability for subjects who are from 3 to 17 years old at all dose levels, and can trigger a strong immune response against the COVID-19 after being vaccinated with two doses.

The results of this study come from a randomized, double-blind, placebo-controlled phase I/II clinical trial evaluating the safety, tolerability, and immunogenicity of the COVID-19 inactivated vaccine in healthy people aged 3-17 years. The study was carried out at the Shangqiu City Liangyuan District Center for Disease Control and Prevention in Henan, China. All participants were randomly assigned, using stratified block randomisation to receive three doses of 2 μg, 4 μg, or 8 μg of vaccine or control 28 days apart.
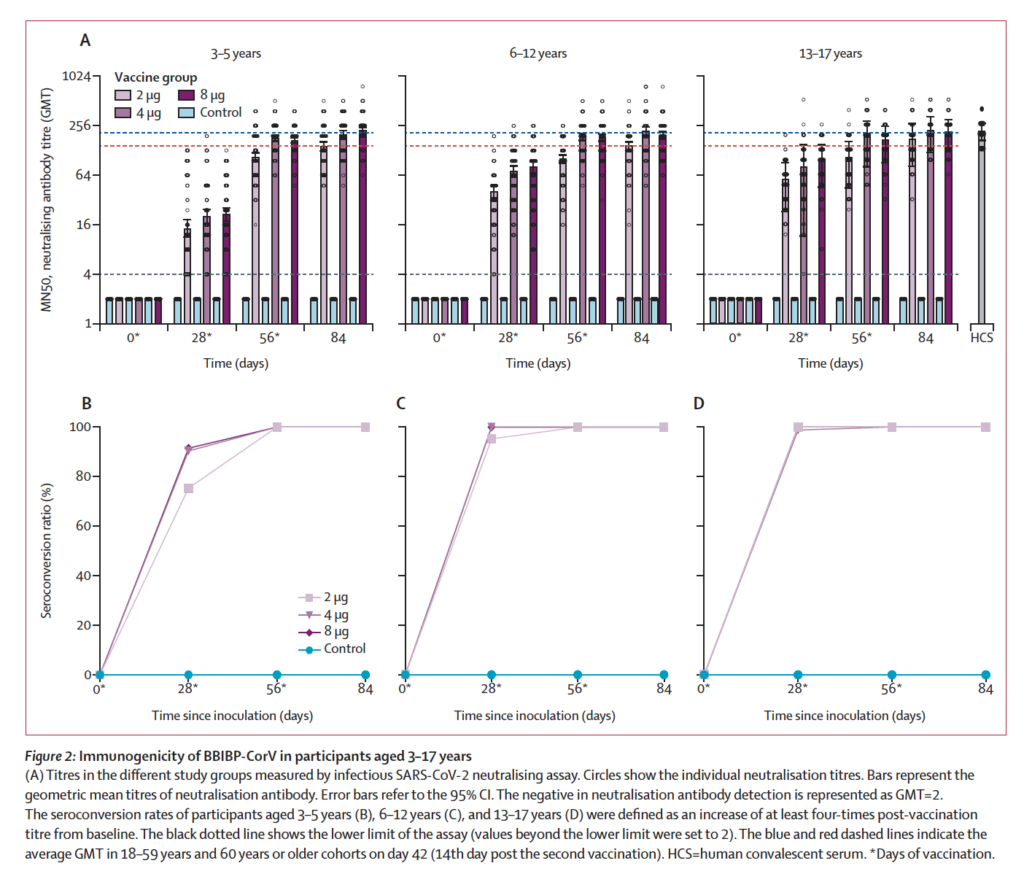
In terms of immunogenicity, after vaccination with the inactivated vaccine according to a 0, 28, and 56-day immunization schedule, the results showed that the immunogenicity of 28 days after 3 doses was higher than 28 days after 2 doses. The anti-coronavirus neutralizing antibody geometric mean titer (GMT) of the target dose group for subjects aged 13-17 years old, 6-12 years old, and 3-5 years old at 28 days after immunization was 199.1, 184.8, and 199.1, respectively.
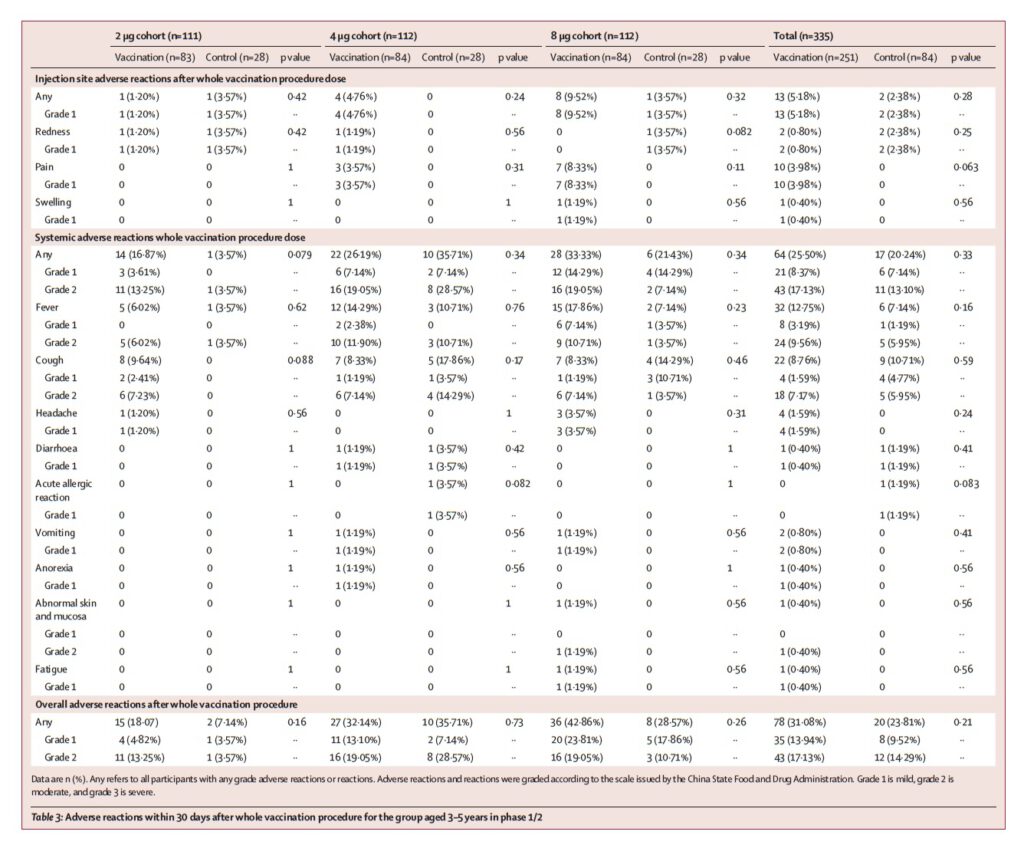
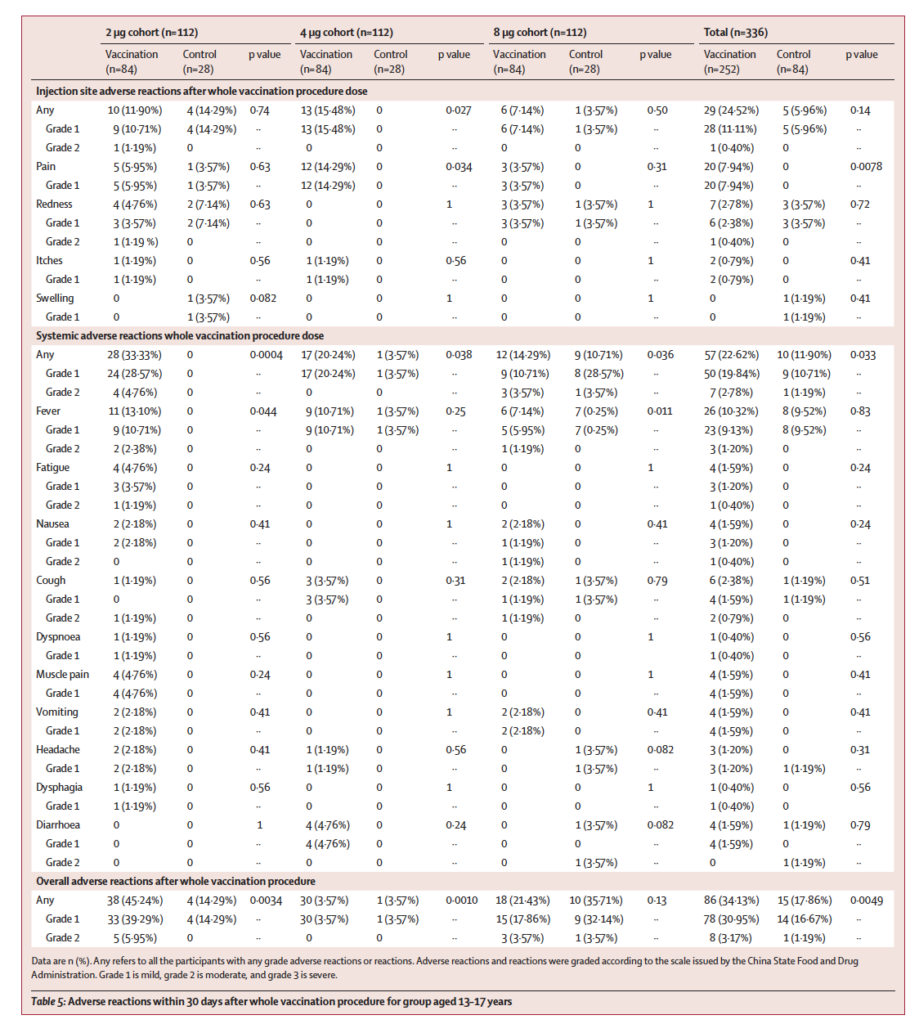
In terms of safety, the main local adverse reaction was pain at the injection site, which happened around 4%, 9.1%, and 7.9% in the 3-5 years old group, 6-12 years old group, and 13-17 years old group, respectively. The systemic adverse reactions were fever, accounting for around 12.7%, 5.2%, and 10.3% in the 3-5 years old group, 6-12 years old group, and 13-17 years old group, respectively, but the severity of adverse reactions is mostly mild.
According to China Biotech, research has shown that the 4-fold growth rate of neutralizing antibodies and IgG antibodies in the low-dose, medium-dose, and high-dose groups 28 days after the full course of immunization is 100%. The GMT levels of neutralizing antibodies and IgG antibodies of the low, medium, and high-dose test vaccines increased significantly at 28 days after the full vaccination; the GMT levels of neutralizing antibodies and IgG antibodies in the middle-dose and high-dose groups were higher than that of the low-dose group in 28 days after the full vaccination.
According to Zhang Yuntao, chief scientist and vice-president of Sinopharm subsidiary CNBC, after the conditional marketing approval of Sinopharm China’s COVID-19 inactivated vaccine, the expanded clinical trials for the 3-17-year-old population were continued after adult use. Based on the results from expanded clinical trials, there was no significant difference in the positive conversion rate compared with the adult group, and no serious adverse reactions were seen.
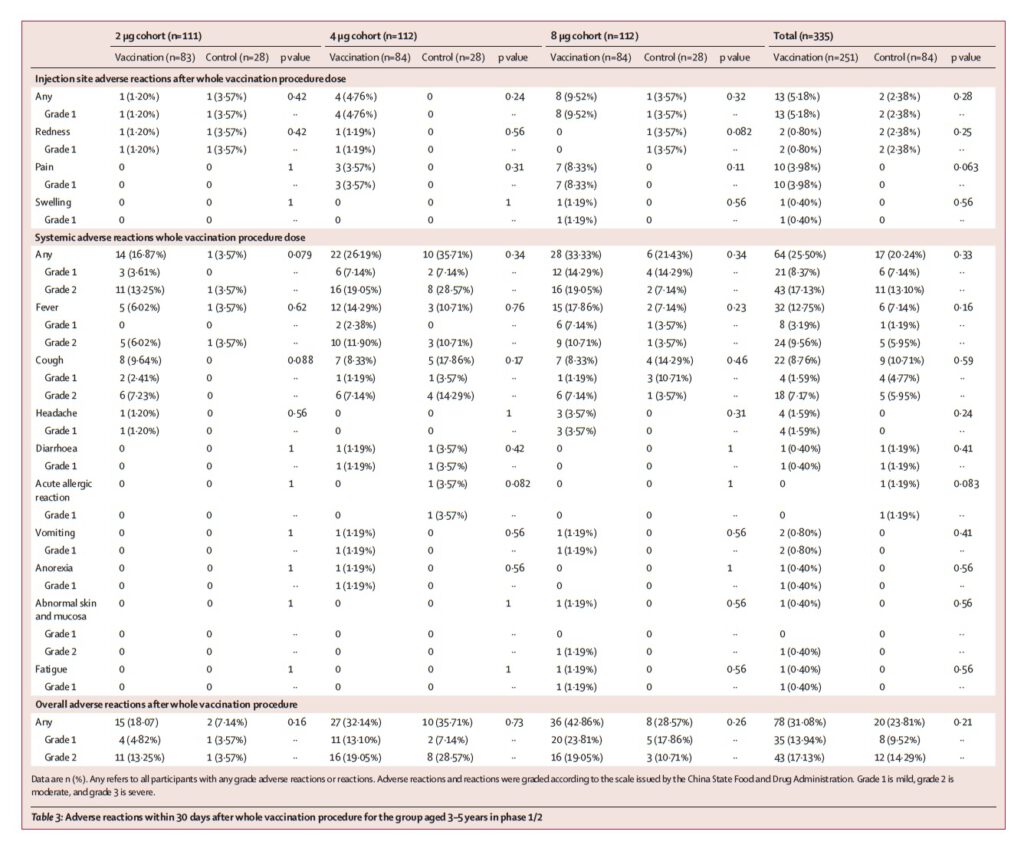
“The safety data meets the expected requirements. Compared with the adult group, there is no increased safety risk, mainly local pain and systemic fever, mainly some first-level adverse reactions, which are fully in line with expectations. The immunogenicity meets the requirements. The data formed during the phase I/II clinical study proves that the vaccine can be used in the age group of 3-17 years,” confirmed him.
“To ensure safety, this experiment started from 13-15 years old, pushed down to 6-12 years old, and then pushed down to 3-5 years old. The development of the immune system of children and adolescents is not necessarily mature. People of this age group are still growing. To be responsible, we adopted the method of inverting the age group and systematically completed the phase I/II clinical study. After obtaining the data, in April, we submitted the relevant data to the China National Food and Drug Administration,” addressed Zhang.
On 6 June, the immune bridging clinical trial of the Chinese COVID-19 inactivated vaccine developed by the CNBC in the 3-17-year-old group was launched in Abu Dhabi, UAE, evaluating immunogenicity and safety after vaccination in healthy people among 900 people of different nationalities aged 3-17. And the final results show that the security is relatively high, pointed out Zhang.
According to the information previously released by CNBC, its COVID-19 inactivated vaccine was approved in Bahrain for children aged 3-11 with complications and weakened immunity, as well as people aged 12-17; the UAE Ministry of Health and Prevention also approved to use CNBC vaccines for children aged 3-17 in the country. In China, on 16 July 16, this CNBC COVID-19 inactivated vaccine was approved for emergency use in people aged 3-17 as well.
(Source: the Lancet infectious diseases)



